Human trials of Coronavirus vaccine to begin in UK and Germany
As global cases soared past 2.5 million, the race is on to develop a vaccine to help contain the spread of Corona Virus.
There are 86 teams across the world currently working to develop a Covid-19 vaccine, including a handful at the clinical trial stage.
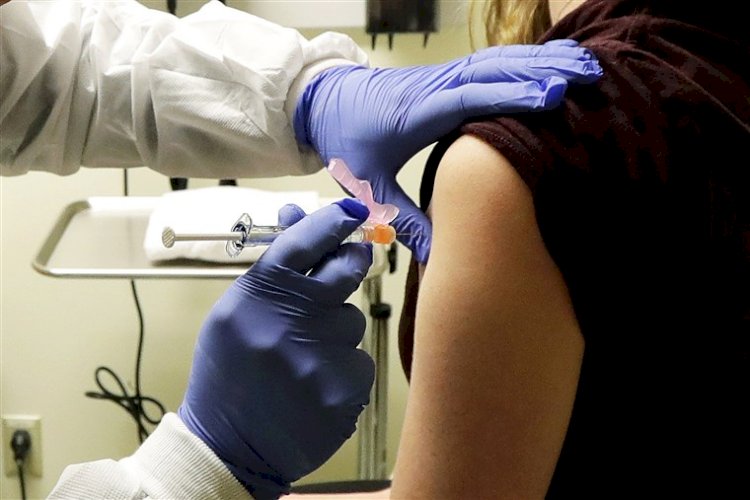
British Health Minister Matt Hancock has announced that a coronavirus vaccine developed by researchers at Oxford University will be tested on people from this Thursday.
However Matt Hancock said that there was nothing about this process that was certain. He said that the vaccine from the Oxford trial will be tested in people starting Thursday. The vaccine being developed by scientists at the University of Oxford believe that thee is an 80 per cent chance of success.
The Oxford study will begin testing on 510 volunteers between 18 and 55 years of age. They will receive injections of a small part of the coronavirus code, packaged into a harmless virus. This is designed to spark a natural immune response and provide some immunity if the person is then exposed to the coronavirus. Volunteers will be monitored for six months, with some to be given a second vaccine shot in four weeks’ time.
The trial is to be expanded to volunteers aged between 55 to 70 years, and then later moving on to the over-70 age group. A randomly-selected control group will be vaccinated against meningococcal disease.
Phase 3 of the trial will involve as many as 5000 people.
The Oxford study, led by Professor Sarah Gilbert, has made bold claims that the first batches of a vaccine it has called ChAdOx1 nCoV-19 could be made available in six months if the trial is successful.
Germany follows the UK in approving a human trial of Corona Virus vaccine.
A clinical test of a Covid-19 vaccine in Germany has been approved, according to the country’s Federal Institute for Vaccines.
The trial will see 200 healthy participants between the age of 18 and 55 receive several variants of the vaccine, developed by German biotech company BioNTech, as scientists examine its efficacy in providing immunity against the virus.
Additional tests will be conducted on more people, including those at higher risk from the disease, in a second stage.
BioNTech said it was developing the vaccine candidate, named BNT162, together with pharmaceutical giant Pfizer.
BNT162 is also set to be trialled in the US, once regulatory approval for testing on humans had been secured there.
In China, early-stage human tests for two experimental vaccines were approved earlier this month, according to state media Xinhua.
The Chinese vaccine candidates are being developed by a Beijing-based unit of Nasdaq-listed Sinovac Biotech and by the Wuhan Institute of Biological Products, an affiliate of state-owned China National Pharmaceutical Group.




 mode1
mode1